A single experimental antibody could halt triple-negative breast cancer’s ruthless advance by precisely targeting a protein that fuels its deadliest traits.
Story Snapshot
- MUSC researchers developed a humanized monoclonal antibody targeting SFRP2 protein, slowing TNBC tumor growth and metastases in mouse models.
- The antibody reactivates immune cells like M1 macrophages and T-cells, killing chemo-resistant cells without harming healthy tissues.
- Licensed to Innova Therapeutics on January 22, 2026, for clinical trials after nearly two decades of research starting in 2008.
- Offers hope for TNBC patients, who face 40-50% recurrence rates and poorer survival, especially younger women and Black communities.
- Distinguishes itself from broad chemotherapies and ADCs by focusing on immune retraining and tumor-specific action.
SFRP2 Protein Drives TNBC Aggression
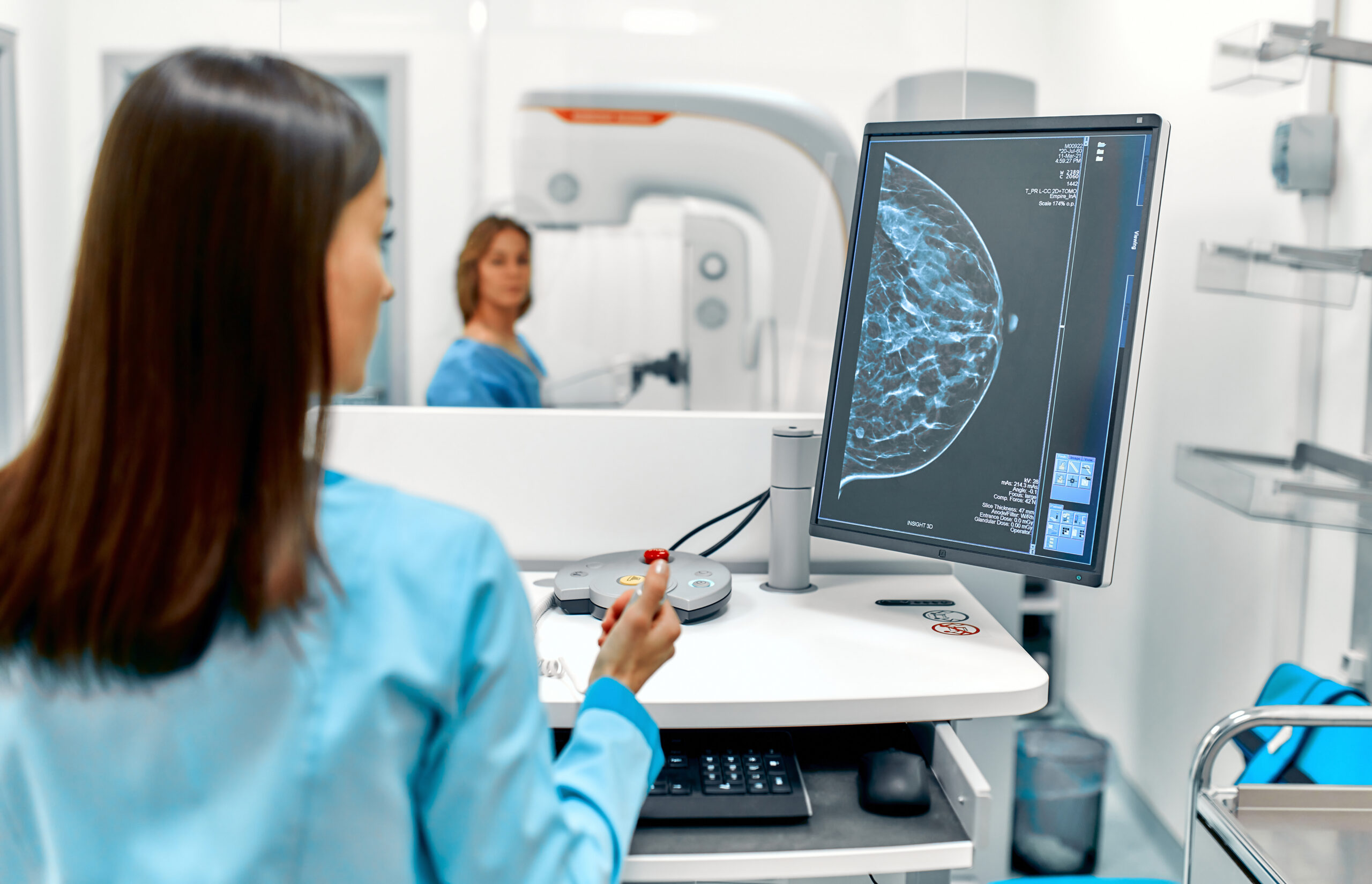
Triple-negative breast cancer lacks estrogen, progesterone receptors, and HER2 protein, comprising 15-20% of cases with high relapse and metastasis risks. MUSC Hollings Cancer Center scientists identified SFRP2 in 2008 as a key driver. This secreted frizzled-related protein activates Wnt signaling to promote tumor growth. SFRP2 also polarizes macrophages to pro-tumor M2 states and exhausts T-cells, enabling immune evasion and chemotherapy resistance. TNBC’s 77% five-year survival lags behind 93% for other subtypes, with lung metastases signaling dire outcomes.
Antibody Development Targets Tumor Microenvironment
Nirag C. Klauber-DeMore, MD, led the charge at MUSC, engineering a humanized monoclonal antibody specific to SFRP2. In preclinical mouse models of primary and advanced TNBC, the antibody slowed primary tumor growth by over 60%. It reduced lung metastases dramatically. The treatment killed persistent chemotherapy-resistant cells that standard doxorubicin failed. Immune reactivation shifted macrophages to anti-tumor M1 phenotypes and boosted T-cell activity. Critically, the antibody avoided accumulation in healthy organs, minimizing toxicity risks.
Watch:
Preclinical Results Outshine Current Standards
Current TNBC treatments rely on chemotherapy like doxorubicin and antibody-drug conjugates such as sacituzumab govitecan (Trodelvy). ASCENT-04 trial data showed Trodelvy plus pembrolizumab superior in first-line PD-L1-positive metastatic TNBC. Yet resistance limits broad efficacy, especially in non-PD-L1 cases. This SFRP2 antibody addresses chemo-resistance directly and reactivates exhausted immunity. Lead researcher Klauber-DeMore notes its effectiveness even when standard therapies fail, aligning with common-sense priorities for targeted, less toxic options over blunt chemo.
Lillian Hsu, MD, highlights how the antibody pushes macrophages to the beneficial M1 state without toxic effects. Team member Julie Siegel contributed to validation testing. These results position the therapy as a potential complement to ADCs and immunotherapy combos.
This new antibody may stop one of the deadliest breast cancers https://t.co/DqK7SHUI7f
— #TheRebelDemocrat (@ejnyamogo) January 23, 2026
Licensing and Path to Human Trials
On January 22, 2026, MUSC published findings in Breast Cancer Research and licensed the antibody to Charleston-based Innova Therapeutics, co-founded by Klauber-DeMore. Innova now pursues funding for Phase 1 trials. FDA granted Rare Pediatric Disease and Orphan Drug designations for SFRP2-linked osteosarcoma, providing incentives. This academic-to-biotech handoff leverages nearly 20 years of mechanistic insights, from SFRP2 discovery to humanization.
Implications for Patients and Industry
TNBC disproportionately strikes younger women and Black patients, fueling recurrence rates of 40-50%. Success here could shift paradigms toward immune-retraining precision therapies, integrating with frontline options like Trodelvy expansions. Economic boosts hit Charleston biotech; socially, fewer chemo side effects enhance quality of life. Politically, FDA incentives underscore support for unmet needs in aggressive cancers. Preclinical promise is strong, though human translation carries standard risks—yet mouse specificity encourages optimism rooted in facts.
Your instant doctor companion – online 24 hours a day.
Sources:
This new antibody may stop one of the deadliest breast cancers
New England Journal of Medicine Publishes Phase 3 ASCENT-04/Keynote-D19 Results Supporting Trodelvy® (sacituzumab govitecan-hziy) Plus KEYTRUDA® (pembrolizumab) as a Potential New Standard of Care in First-Line PD-L1+ Metastatic Triple-Negative Breast Cancer
FDA Grants Priority Review to Gedatolisib for Advanced Breast Cancer
The Science Behind Hope: 5 Breakthrough Areas in Breast Cancer Research from 2025
ADC Combination Outperforms Standard Treatment of Advanced Triple-Negative Breast Cancer
Experts Forecast Cancer Research and Treatment Advances in 2026