AI technology now predicts breast cancer risk 2.2 times more accurately than old questionnaire methods, offering American women a powerful tool for early prevention.
Story Highlights
- Prognosia Inc.’s AI analyzes mammograms using only images and age for superior five-year risk prediction, earning FDA Breakthrough Device designation.
- Washington University researchers developed the software, licensed to Prognosia, compatible with 2D and synthetic 3D mammograms for broad use.
- Hologic’s Genius AI caught 32% of cancers radiologists missed in 7,500 exams, boosting detection amid radiologist shortages.
- $16M PRISM trial tests AI in real-world screening across five states, promising fewer false positives and recalls.
Breakthrough in Breast Cancer Risk Prediction
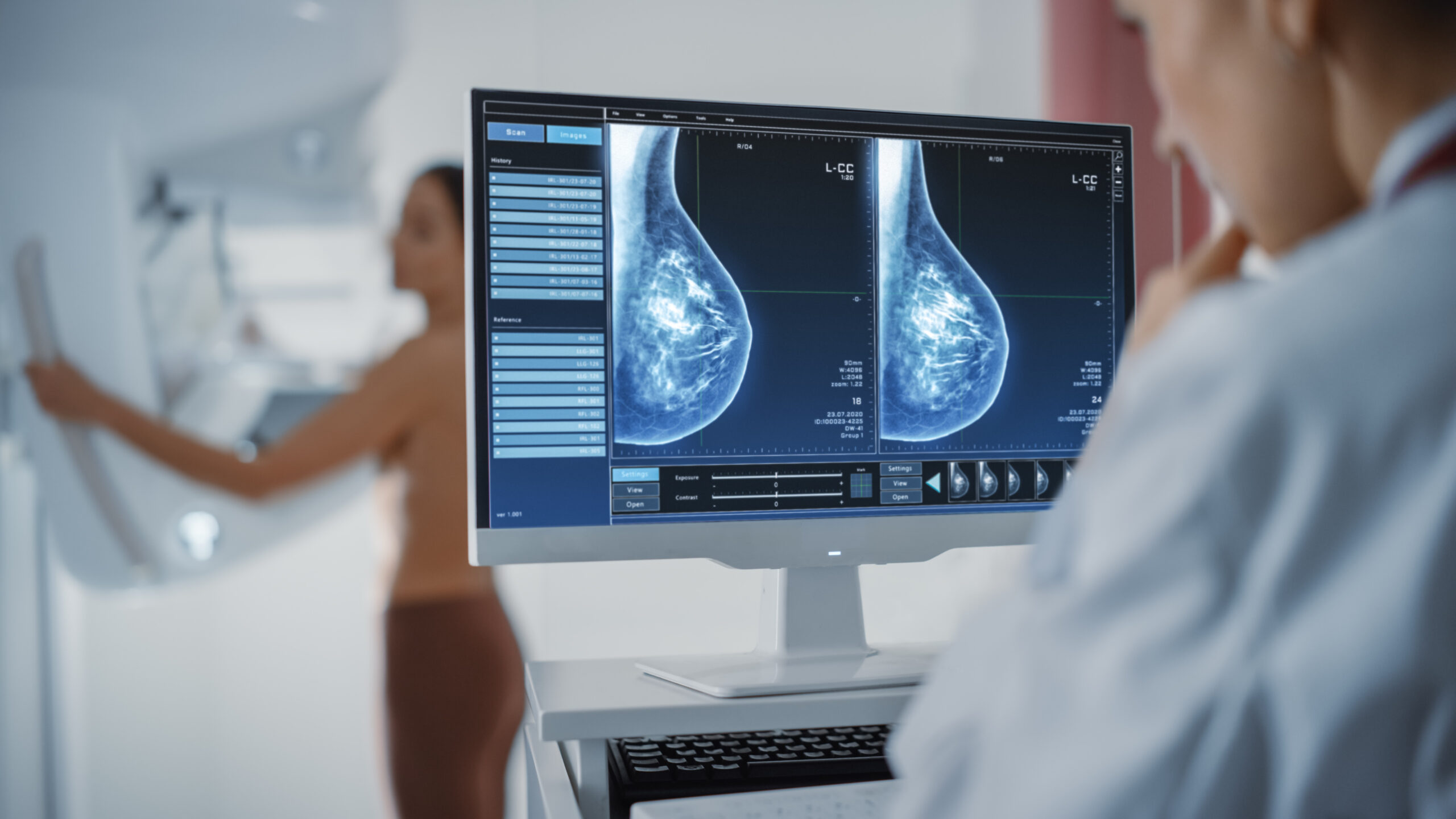
Researchers at Washington University School of Medicine in St. Louis created AI software licensed to Prognosia Inc. This tool examines mammograms to forecast a woman’s five-year breast cancer risk. It outperforms traditional models like the Breast Cancer Surveillance Consortium calculator by 2.2 times. The system relies solely on mammogram images and age, eliminating subjective questionnaires on race and family history. FDA granted Breakthrough Device designation, speeding potential approval for U.S. clinics. This advances objective, efficient screening for women ages 50-74.
Proven Gains from AI in Detection Trials
Hologic’s Genius AI Detection software flagged 32% of breast cancers missed by radiologists in a 2025 Mass General Brigham study of 7,500 retrospective exams. It identified 90% of known cancers with precise localization for interventions. A 2020 Nature study showed AI cutting false positives by 6% in the U.S. across 91,000 mammograms. Sweden’s Lancet Oncology trial found AI detecting 20% more cancers than radiologists alone in over 80,000 women. These results highlight AI’s role in easing overburdened radiology workflows.
A smarter way to screen for breast cancer is emerging: https://t.co/bJmtsgVKT9
— Ken Gusler (@kgusler) January 4, 2026
Dr. Manisha Bahl, study lead, called the findings promising for redefining detection. Radiologist Amy K. Patel noted AI improves over time and aids beyond mammograms, like DCIS risk assessment. Such tools support self-reliant healthcare, aligning with conservative priorities for innovation without bureaucratic delays.
Ongoing Trials and Stakeholder Momentum
UC Davis Health and UCLA Health co-lead the $16M PRISM trial, funded by PCORI in 2025 across California, Florida, Massachusetts, Washington, and Wisconsin. The study randomizes mammograms to AI-assisted versus standard reads, testing reductions in false positives and recalls. Diana Miglioretti, UC Davis data center lead, described it as the first large-scale U.S. randomized AI trial. Siteman Cancer Center plans a trial integrating Prognosia’s risk AI with routine screening for high-risk referrals.
Graham Colditz from Washington University emphasized global rollout potential using existing mammograms. These efforts prioritize patient outcomes and efficiency. Developers like Prognosia and Hologic seek commercialization, while academics focus on evidence-based validation. FDA’s role accelerates tools that empower families with better prevention options.
Watch:
Impacts on Families and Healthcare Efficiency
Short-term benefits include fewer unnecessary recalls and reduced patient anxiety for the over 75% of women ages 50-74 complying with biennial screening guidelines. Long-term, personalized risk scores enable earlier interventions, especially for underserved communities. Economic signals show promise with $16M trial investments and $40-50 add-on fees for tools like Hologic’s AI. Insurers may cover costs post-trials, easing family burdens.
Social equity grows if AI performs across demographics, though experts caution needs for diverse data validation on cancer types like lobular. Broader effects shift healthcare to AI augmentation, reducing radiologist shortages without replacing human judgment. This common-sense progress supports traditional family health protections under President Trump’s pro-innovation leadership.
Your instant doctor companion – online 24 hours a day.
Sources:
AI-based breast cancer risk technology receives FDA Breakthrough Device designation
Artificial Intelligence in Breast Cancer Screening
UC Davis Health to Co-Lead $16 Million Study Examining AI’s Role in Reading Mammograms
When AI Sees What Radiologists Miss: How Artificial Intelligence is Reshaping Breast Cancer Detection
Studying Artificial Intelligence in Breast Cancer Screening
Hologic’s AI-Powered Mammography Technology Flagged a Third of Breast Cancer Cases Initially Interpreted as Negative
Mammogram AI predictive models for both breast cancer, heart disease
UCLA to lead $16 million national study on artificial intelligence
First major trial of AI in breast cancer screening launches in the USA